Wavelength From Mass And Velocity
De Broglie Wavelength Formula
De Broglie Wavelength Formula
In some situations, light behaves like a wave, while in others, it behaves like particles. The particles of calorie-free are called photons, and they can exist idea of as both waves and particles. Louis de Broglie (1892-1987) developed a formula to relate this dual wave and particle beliefs. It tin can also be applied to other particles, like electrons and protons. The formula relates the wavelength to the momentum of a wave/particle.
For particles with mass (electrons, protons, etc., but not photons), there is another form of the de Broglie wavelength formula. At not-relativistic speeds, the momentum of a particle is equal to its rest mass, m, multiplied by its velocity, 5.
The unit of measurement of the de Broglie wavelength is meters (m), though it is ofttimes very small, and then expressed in nanometers (1 nm = 10(-9) m), or Angstroms (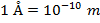 ).
).
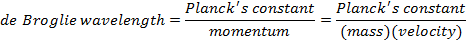
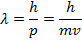
λ = the de Broglie wavelength (one thousand)
h = Planck's abiding (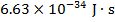 )
)
p = momentum of a particle (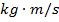 )
)
thousand = mass of a particle (kg)
five = velocity of a particle (m/s)
De Broglie Wavelength Formula Questions:
1) A certain photon has momentum 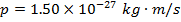 . What is the photon'south de Broglie wavelength?
. What is the photon'south de Broglie wavelength?
Answer: The de Broglie wavelength of the photon can be constitute using the formula:
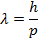
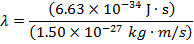
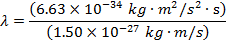
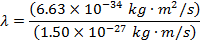
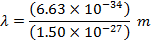
λ = four.42 10 x(-seven) m
λ = 442 10 10(-9) m
λ = 442 nm
The de Broglie wavelength of the photon is 442 nm. This wavelength is in the bluish-violet part of the visible light spectrum.
2) The de Broglie wavelength of a certain electron is 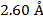 . The mass of an electron is mdue east = 9.109 x 10(-31) kg. What is the magnitude of the velocity of this electron?
. The mass of an electron is mdue east = 9.109 x 10(-31) kg. What is the magnitude of the velocity of this electron?
Respond: The magnitude of the velocity of this electron can be institute past rearranging the de Broglie wavelength formula.
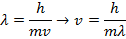
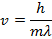
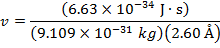
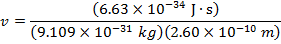
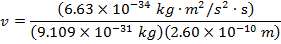
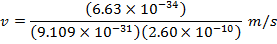
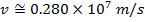
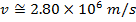
The electron with de Broglie wavelength 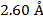 has a velocity value of 2.80 x 106 m/southward.
has a velocity value of 2.80 x 106 m/southward.
Wavelength From Mass And Velocity,
Source: https://www.softschools.com/formulas/physics/de_broglie_wavelength_formula/150/
Posted by: brousseauvedge1990.blogspot.com


0 Response to "Wavelength From Mass And Velocity"
Post a Comment